Cannabis and THC: Neurotoxic or Neuroprotective?
By Kenneth Anderson, MA
Normal and Retrograde Neurotransmission
Delta-9 THC (delta-9-tetrahydrocannabinol) and CBD (cannabidiol) are the two most commonly found cannabinoids in the cannabis plant. The plant contains over 100 other cannabinoids as well. THC and CBD both affect the endocannabinoid system. “Endo-” is a Greek prefix meaning “inside.” “Endocannabinoid system” refers to all the systems of the body which contain cannabinoid receptors and are affected by cannabinoids. We will be primarily focused on the effects of cannabinoids in the central nervous system, and on the CB1 receptor, which is the main cannabinoid receptor there.
Drugs like THC can affect the central nervous system because there are also naturally occurring cannabinoids in the central nervous system, the endogenous cannabinoids, aka endocannabinoids. They are produced by the human body itself; “endognous” means “occuring within.” The two most common endocannabinoids are 2-AG (2-arachidonoylglycerol) and AEA (anandamide). Drugs like THC are referred to as exogenous cannabinoids. “Exo-” is a Greek prefix meaning “outside.” Exogenous drugs are produced outside the human body, by plants, animals, or artificial synthesis.
Normally, neurotransmission takes place when a neurotransmitter is released from an axon, travels across the synaptic gap, and binds to a receptor on the dendrite of another neuron. This is how neurotransmission with most neurotransmitters (e.g., GABA, glutamate, dopamine, serotonin, etc.) works. An example of this type of neurotransmission, with a glutamate-releasing (i.e., glutamatergic) neuron, is given in Figure 1. In response to the electrical signal sent down the axon, the glutamate vesicles in the axon terminal burst and glutamate travels across the synaptic cleft to bind with the glutamate receptors in the dendrite, increasing the electrical potential in the dendrite.
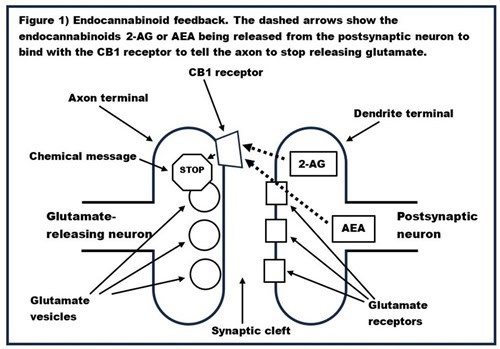 However, neurotransmission of endocannabinoids goes in the opposite direction; the endocannabinoid is released from the dendrite and binds to a receptor on the axon. This is called retrograde neurotransmission. This retrograde neurotransmission of the endocannabinoids acts as a brake on normal neurotransmission. Figure 1 also shows how the endocannabinoids 2-AG or AEA can be released from the dendrite to bind with the CB1 receptor on the axon terminal, which sends a chemical message to slow down the release of glutamate. The same process occurs with neurons that release other neurotransmitters.
However, neurotransmission of endocannabinoids goes in the opposite direction; the endocannabinoid is released from the dendrite and binds to a receptor on the axon. This is called retrograde neurotransmission. This retrograde neurotransmission of the endocannabinoids acts as a brake on normal neurotransmission. Figure 1 also shows how the endocannabinoids 2-AG or AEA can be released from the dendrite to bind with the CB1 receptor on the axon terminal, which sends a chemical message to slow down the release of glutamate. The same process occurs with neurons that release other neurotransmitters.
In other words, the main job of endocannabinoids is to act like the flyball governor on a steam engine or like a thermostat; they are a feedback mechanism to slow down neurotransmitter release so that too much is not released. Why would it be valuable for endocannabinoids to regulate the amount of neurotransmitters released? Let’s take a look at glutamate. Glutamate is the brain’s primary excitatory neurotransmitter. In other words, when glutamate binds to the receptors on the postsynaptic neuron, it makes it more likely that the postsynaptic neuron will fire. However, if the postsynaptic neuron fires too frequently it will die. This is called excitotoxicity. One job of the endocannabinoid is to prevent excitotoxicity. Likewise, GABA is the brain’s primary inhibitory neurotransmitter; GABA makes the postsynaptic neuron less likely to fire, and too much GABA slows things down too much. In the case of neurons which release GABA into the synapse, the endocannabinoid prevents too much GABA from being released so that things do not slow down too much. The endocannabinoid system is the synapse’s feedback mechanism; it prevents things from going too fast or too slow and ensures that they function just right. If glutamate is the accelerator and GABA is the brake, then endocannabinoids are the governor.
Neuron Death and Birth (Neurogenesis)
Before we go on to look at data on whether THC is neurotoxic or neuroprotective, two other issues should be addressed. The first is the natural rate of neuron death and the second is neurogenesis. According to Solveig et al. (2014), in one single human brain, 85,000 neurons die every day. So, when we discuss neurotoxicity, we are actually discussing an increase over the natural rate of neuronal death. Secondly, although other cells in the body regenerate, for many years it was believed that neurons in adult organisms did not regenerate. In recent years, this has been proven false, as it has been found that neurons in certain specific brain areas, such as the hippocampus (which is the seat of memory), regenerate in rats and a number of other animals. However, it remains a matter of controversy whether this adult neurogenesis also occurs in humans.
A 2007 article by Gilbert et al. investigated the neuroprotective effect of THC on a laboratory culture of hippocampal neurons from the rat. The researchers found that reducing the number of magnesium ions in the hippocampal neuron culture led to death from excitotoxicity of nearly all the neurons in the culture. However, if THC was also added to the culture, about 84% of neurons survived the depletion of magnesium ions, showing a strong neuroprotective effect of THC on hippocampal neurons.
A 2018 paper by Suliman et al. reported that low doses of THC increased neurogenesis in rat hippocampal neurons, and also enhanced learning and memory in rats. The rats in this experiment were 64 four-week-old juvenile male Sprague Dawley rats. The rats were divided into two groups, one group received THC treatment for seven days, and one group received THC treatment for 21 days. These two groups were further subdivided into four groups each: one group received 0.0 mg/kg THC, one received 0.75 mg/kg THC, one received 1.5 mg/kg THC, and one received 3.0 mg/kg THC. Hence, there were a total of eight groups. The THC was injected into the abdomen, presumably once per day. By way of comparison, 0.15 to 0.30 mg/kg THC is a mild dose for humans, and 0.45 to 0.6 mg/kg is a strong dose for humans.
On the final day of treatment the rats were tested for memory and cognitive ability. The short-term memory of the rats was tested using the novel object recognition test. In this test, the rat is placed in a box for five minutes with two identical objects, for example two cylinders. The rat is then removed from the box for one minute, and one of the objects is replaced with a novel object, for example, one of the two cylinders might be replaced with a cube. The amount of time that the rat spends exploring the novel object is indicative of short-term memory. The longer the rat explores the novel object, the better the short-term memory. The identical test is used to measure long-term memory; the only difference is that instead of removing the rat from the box for one minute before returning it, the rat is removed for one day. Rats treated with 1.5 mg/kg of THC performed significantly better than rats in the other three groups on both short-term memory and long-term memory. The rats which had received no THC performed the worst on this test. There was no difference between the rats which had been treated for seven days and those which had been treated for 21 days.
After testing for memory and cognitive ability the rats were euthanized, and the number of neurons in their hippocampus was measured. Examination of the rats’ brains with several chemical tests showed that there were significantly more hippocampal neurons in the brains of the rats treated with 1.5 mg/kg THC than the rats in the other three treatment conditions. Rats which received no THC showed the fewest new neurons. Some of the chemical tests indicated significantly more neurogenesis for the rats treated for 21 days compared to those treated for seven days; however, the other chemical tests showed no significant difference based on length of treatment.
On the other hand, a 1998 study by Chan et al. showed that THC was toxic to a laboratory culture of newborn Sprague Dawley rat hippocampal neurons. The researchers found that exposing the culture to one micro mole per liter of THC for six days killed 50% of the neurons in the culture. One micro mole per liter is a typical recreational dose of THC for humans. However, there is a great difference between a cell culture and a living organism; in a living organism, THC is quickly metabolized and eliminated, whereas in a cell culture it lasts indefinitely. Nevertheless, a 1998 study by Landfield et al. showed that high doses of THC in living rats decreased the number of neurons on the hippocampus, although the behavioral effects were minimal.
Silva published a review of neurotoxic effects of THC in 2023. Numerous studies of rats and humans showed learning or memory deficits during and after THC intoxication, as well as hippocampal neuron death. However, this review chose to report only negative effects of THC and excluded any published studies reporting positive effects of THC.
Conclusions: Cannabis and THC – Neurotoxins or Neuroprotectors?
The effects of THC are complex. More study is needed. Findings in rats may not generalize to humans, and findings from adults may not generalize to teens. Nevertheless, the evidence to date suggests that occasional recreational use of cannabis by adults is probably quite harmless, and may confer some benefit. Presumably occasional recreational cannabis users would agree. On the other hand, the neurotoxicity that would likely arise from heavy daily use could turn you into Cheech and Chong.
